Hydrolysis of Condensation Polymers
Hydrolysis is the cleavage of bonds in functional groups by reaction with water. This reaction occurs mainly in polymers that take up a lot of moisture and that have water-sensitive groups in the polymer backbone. Some synthetic polymers that degrade when exposed to moisture include polyesters, polyanhydrides, polyamides, polyethers, and polycarbonates. The rate of hydrolytic degradation can vary from hours to years depending on the type of functional group, backbone structure, morphology and pH. Polymers that readily degrade in the presence of water include polyanhydrides, aliphatic polyesters with short midblocks like polylactic acid and certain poly(amino acids) like poly(glutamic acid). Assuming similar midblocks, the rate of hydrolysis decreases in the order anhydride > ester >> amide >>> ether.
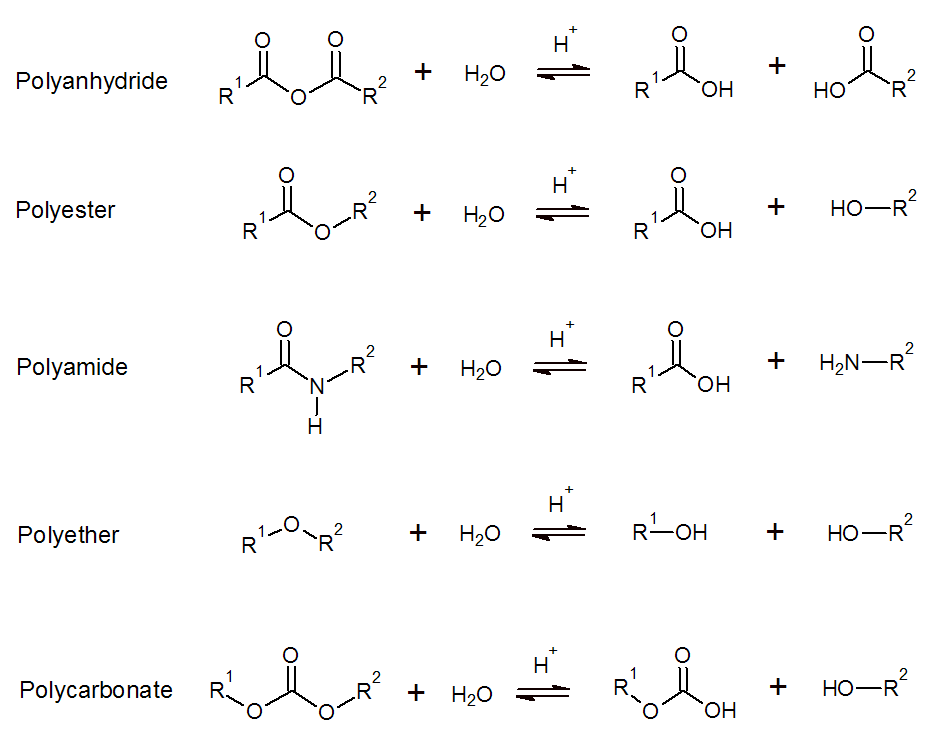
The hydrolysis of semicrystalline polymers such esters, amides and anhydrides occurs usually in two stages; during the first stage, the degradation occurs by diffusion of water into the amorphous regions with subsequent hydrolysis. The second stage starts when moisture penetrates and degrades the crystalline regions. Thus, a bimolecular weight distribution is observed at any time during these two stages. The erosion rate usually increases over time due to decreased crystallinity and molecular weight as well as increased water solubility which turns the polymer into a beehive- or spunge-like porous body with only minor dimensional changes. Surface erosion, on the other hand, occurs at a constant erosion rate and does not affect the molecular weight but produces dimensional changes. This mechanism dominates when the polymer in question has a low water uptake and low degradation rate which is the case for many polycarbonates, polyureas, polyethers, and polyether urethanes. Surface erosion generally results in very predictable mass loss rate without compromising the mechanical and structural integrity of the polymeric material.
There are several factors that affect the hydrolytic stability of a polymer. The most important factors are pH, temperature, hydrophobicity, morphology, degree of crystallinity and porosity. Most or all of these factors affect the water permeability and thus the bulk errosion rate. The pH is one of the most important factors because an acid or a base acts as a catalyst, that is, they greatly accelerate the degradation process. The water uptake and oligomer/monomer water solubility is particularly important for polymers that degrade by bulk errosion. These factors depend on the polarity of the polymer. A lower polarity tends to decrease the reaction rate because both the water content in the polymer as well as the water permebability decrease with decreasing polarity of the polymer. Thus, the hydrolytic stability increases in the same order as the hydrophobicity. Consequently, PBT is more stable than PTT and the later is more stable than PET. In the case of aliphatic polyesters, the hydrolytical stability increases with the lengths of the aliphatic portion in the chain. Thus, the stability of the following aliphatic polyesters increases in the order: Poly(propylene adipate) > Poly(propylene succinate) > Poly(propylene sebacate).
Hydrolyis reactions may be also catalyzed by certain enzymes known as hydrolases. These biological catalysts accelerate the reaction rate in living organisms (bacteria, fungi, etc) without undergoing themself any permanent change. The enzymatic hydrolysis of polymeric materials is a heterogenous process that is affected by both the physiochemical properties of the plastic (MW, crosslinking, chemical composition, surface area, porosity, crystallinity etc.) and the inherent properties of the enzyme (activity, solubility, stability, 3-D conformation etc.). Important extrinsic factors include pH, humidity, temperature, oxygen, and nutrient availability etc.
Synthetic polymers particularly susceptible to enzyme, acid, or base catalyzed hydrolysis include aliphatic polyanhydrides like poly(sebacic anhydride) and polyesters with short midblocks such as poly(lactic acid) (PLA), poly(glycolic acid) (PGA), polycaprolactone (PCL), and poly(hydroxybutyrate) (PHB). These biodegradable materials find many uses in pharamceutical and medical products such as resorbable surgical sutures, resorbable orthopedic devices and controlled-release coatings for drug delivery systems.
Besides water, many other polar solvents such as alcohols, amines, and acids may cause cleavage of C-O or C-N bonds.This process is known as solvolysis and if the solvent is an alcohol as alcoholysis.
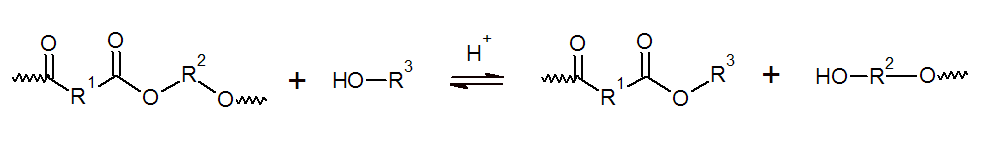
The reaction rate depends on the type of solvolytic agent and its solubility in the polymer as well as on the pH and temperature.