Free Radical Photoinitiators
Photoinitiators are compounds that produce radicals when exposed to UV light. These then react with monomers and / or oligomers to initiate polymer chain growth. They are essential ingredients of all UV-curable adhesives, inks and coatings.
Free radical UV photoinitiators can be classified into Norish
type I and II initiators.
Norish type I
initiators are typically compounds containing benzoyl groups. The carbonyl group of the initiator absorbs a photon
and is transformed into an excited state. Subsequent
homolytic cleavage of the excited α-carbon bond produces two radical
fragments. For instance, the cleavage of
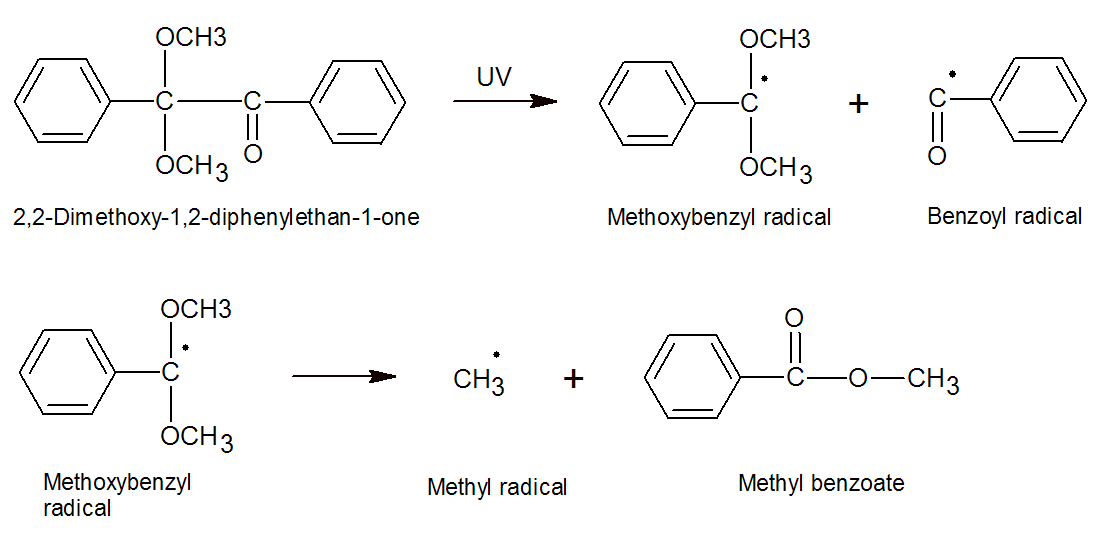
2,2-dimethoxy-1,2-diphenyl-ethan-1-one largely yields a
methoxybenzyl and benzoyl radical. The benzoyl radical initiate free
radical polymerization whereas the methoxybenzyl radical decomposes to give the more stable methyl radical and methyl
benzoate.
Two other very common Norish type I initiators are 1-hydroxycyclohexylphenyl-ketone and 2-hydroxy-2-methyl-1-phenylpropanone. Both are soluble in many solvents (monomers) and can be combined with other initiators.
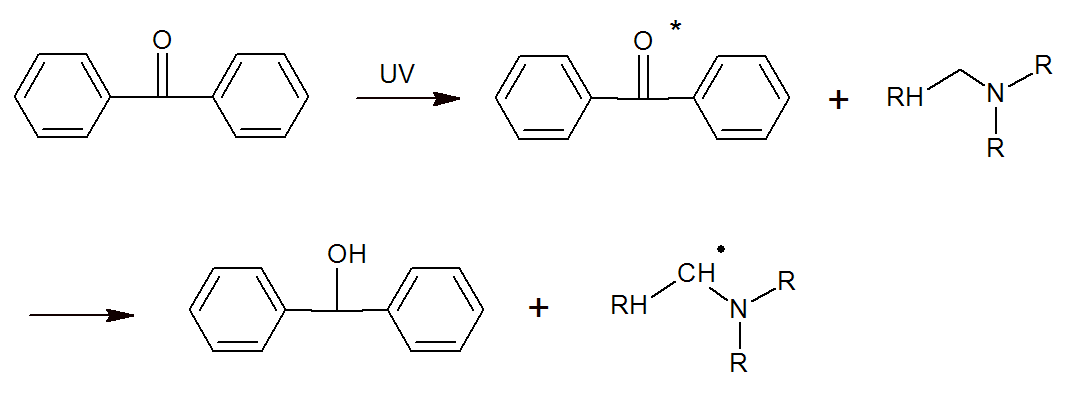
Norish type II initiators absorb UV light to form excited molecules which then abstract an electron or hydrogen atom from a donor molecule (synergist). The donor molecule then reacts with a monomer to initiate polymerization. Common type II photoinitiator systems include benzophenone and its derivative and isopropyl thioxanthone in combination with a synergist such as tertiary amines. The amines function as active hydrogen donors for the excited photoinitiators. The abstraction of hydrogen produces very reactive alkyl-amino radicals which subsequently initiate polymerization.
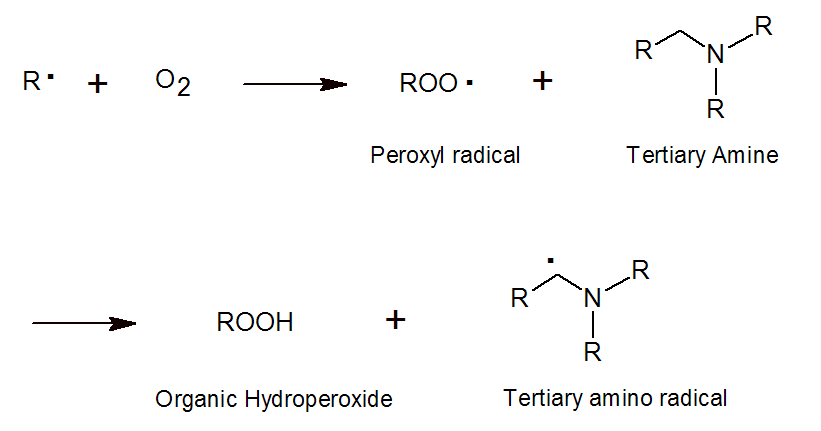
Tertiary amines also reduce air inhibition; oxygen, which diffuses into the mixture, reacts with growing free-radical polymer chains to form unreactive peroxy radicals. The tertiary amines react with these radicals converting them to reactive alkyl-amino radicals, and thereby prevent air inhibition.
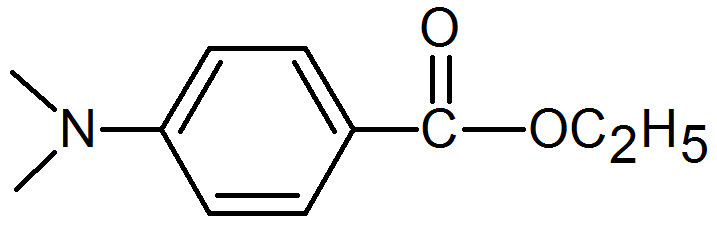
Two frequently used aromatic amine synergists are 2-ethylhexyl-(4-N,N-dimethyl amino)benzoate, and 2-ethyl-(4-N,N-dimethylamino)benzoate.
Another important class of amine synergists is acrylated amines. The acrylate on the amine synergist will allow it to react with the chain radicals and thereby reduce the potential for surface migration (less tack). They also provide faster surface cure, reduced volatility (odor), and increased solvent resistance.
| Compound | Chemical Structure | Type |
|
2,2-Dimethoxy-1,2-diphenylethan-1-one |
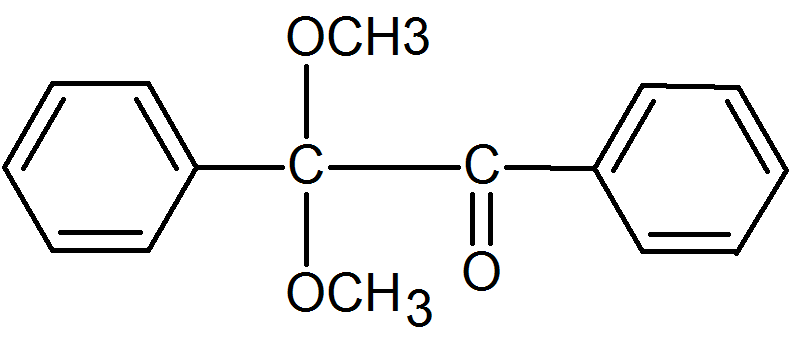 |
α-Cleavage |
|
2-Hydroxy-2-methyl-1-phenylpropanone |
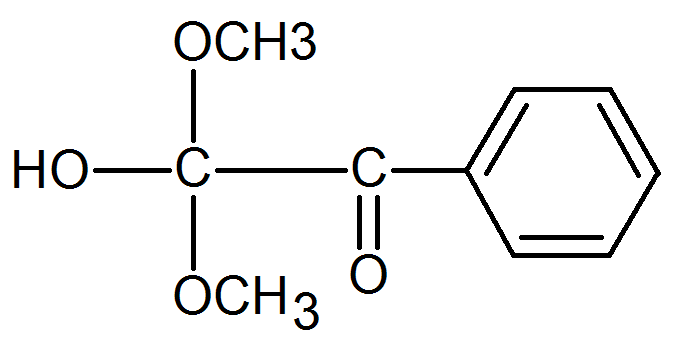 |
α-Cleavage |
|
1-Hydroxy-cyclohexylphenylketone |
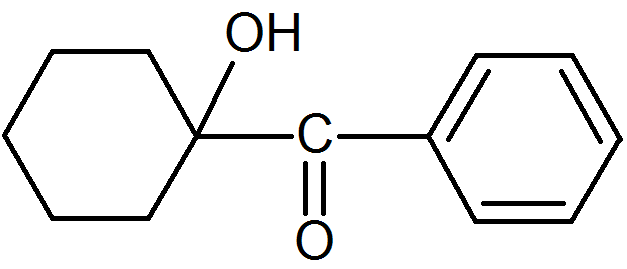 |
α-Cleavage |
|
Benzophenone |
 |
Hydrogen Abstraction |
|
Isopropyl thioxanthone |
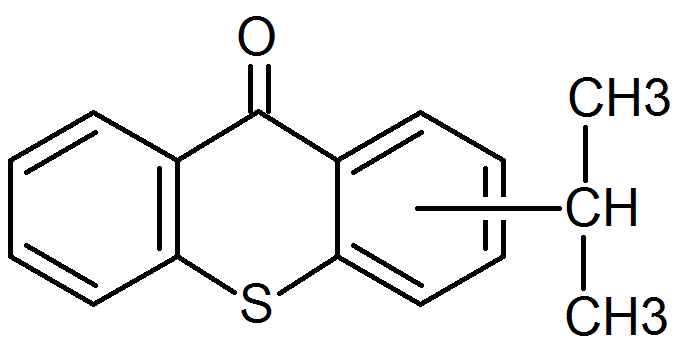 |
Hydrogen Abstraction |
|
2-ethylhexyl-(4-N,N-dimethyl amino)benzoate |
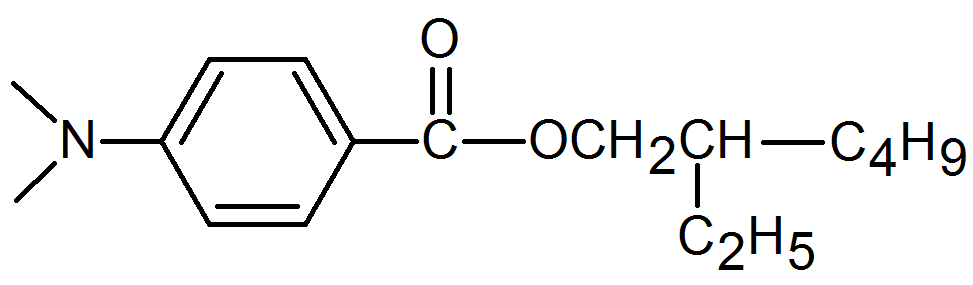 |
Synergist |
|
Ethyl-4-(dimethylamino)benzoate |
 |
Synergist |