Thia-Michael Addition
The thiol Michael addition reaction (also known as thia-Michael addition) is an organic reaction between a thiol and an ene compound with electron-withdrawing group. Like many other ''click'' reactions, this type of reaction can be extremely efficient1 and, therefore, has received significant attention as a postpolymerization modification method, and in the more recent past, for the synthesis of novel polymers. Thiol michael addition reactions have also been utilized to prepare new thioether-based monomers that can be (co)polymerized by a range of established methods.2
The reaction mechanism of the thiol-Michael addition is very similar to that of the even more popular radical-mediated thiol-ene reaction with an anion in place of a radical.3 The reaction starts with a suitable base that abstracts a hydrogen from the thiol. The formed thiolate anion adds to an ene (with electron-withdrawing group) forming an intermediate carbanion, which then abstracts a hydrogen from another thiol to generate a new thiolate anion so that the cycle repeats itself until all double bonds are consumed (see cycle below).3-5, 8
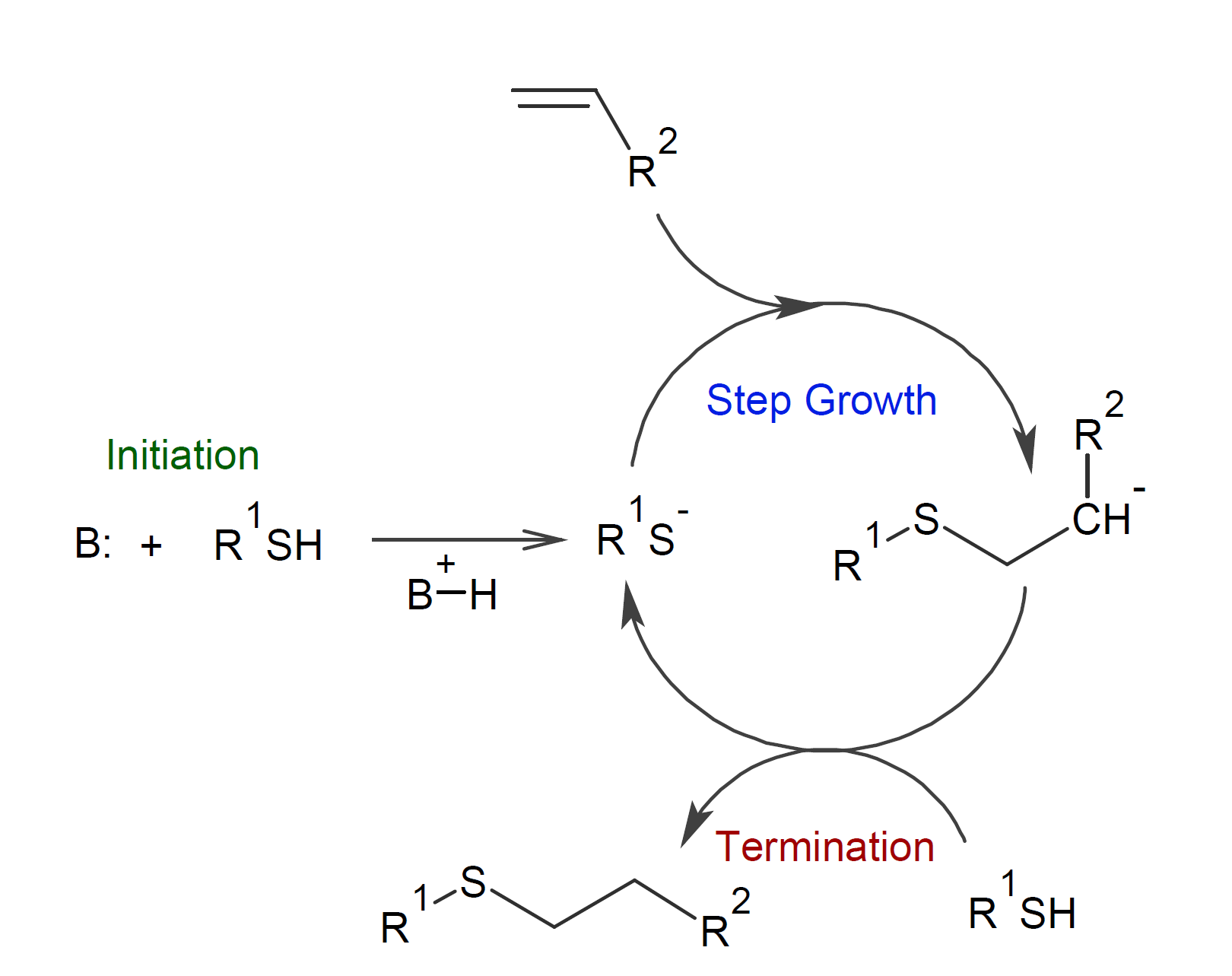
Though the reaction mechanism of a thiol-Michael addition is very similar to that of a thiol-ene reaction, the Michael acceptors show very different reactivities; for example, in a thiol-Michael addition, the reaction rate increases with the electron-deficiency of the Michael acceptor whereas electron rich alkenes are generally favored in radical thiol-ene reactions, i.e. in a Michael addition, α-alkyl or β-alkyl substituted vinyl esters react slower than unsubstituted vinyl esters. For example, methacrylates have typically much lower reactivities than acrylates due to the electron-donating effect and steric hindrance of the methyl group.
Thiols are strong Michael donors and can add to many unsaturaed compounds including norbornene esters, allyl ethers, maleimides, maleates, vinyl ethers, vinyl esters, fatty acid monomers, triallyl isocyanurate, and alkynes. The latter reaction is typically referred to as thiol-yne reaction.2,6
It is important to note that high molecular weight polymers can only be synthesized if both the Michael acceptor and donor are bifunctional or multifunctional monomers. An example of a thia-Michael addition step-growth polymerization that produces polymer chains in this way is the reaction of a bismaleimide with an alkane dithiol (A-A + B-B) such as 4,4'-bis(maleimido)diphenylmethane and 1,6-hexanedithiol which produces poly(imido sulfides). The reaction mechanism of this type of Michael addition is shown below.
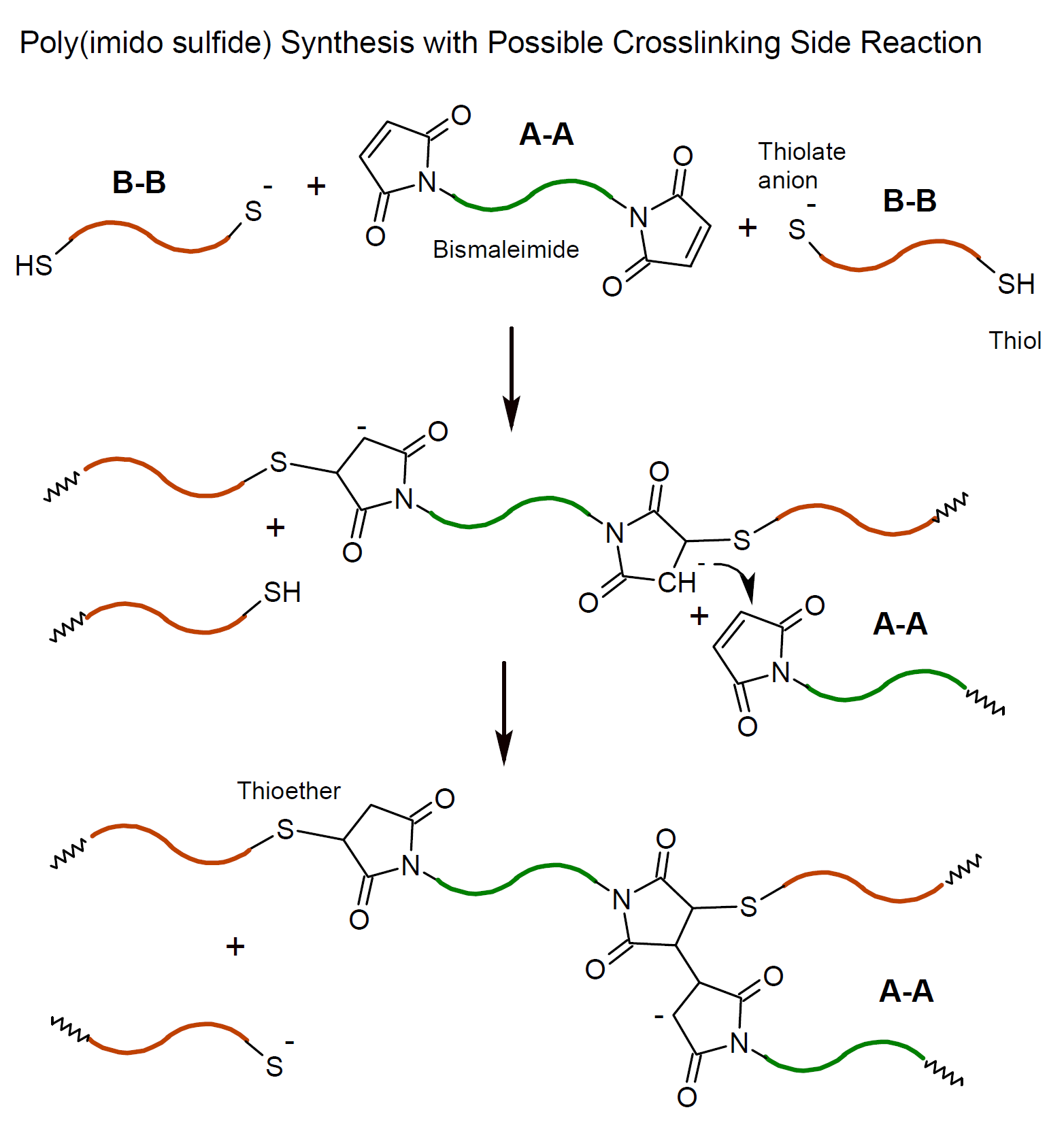
In this particular reaction, carbanions formed upon Michael addition can react with additional maleimide groups or they can abstract a proton from a thioether.7 In the former case, crosslinked polymers are porduced, whereas hydrogen abstraction leads to linear polymers. The use of protic small dithiols monomers favors polymers without crosslinking due to fast protonation of the carbanions.7
Note, the reaction rate strongly depends on the type of ene and decreases with increasing electron density of the carbon–carbon double bond, in other words, thiol-ene reactions favor electron poor enes.5,7
References & Notes
H.C. Kolb, M.G. Finn, K.B. Sharpless, Angew. Chem. Int. Ed., 40, 2005 – 2021 (2001)
A.B. Lowe, Polym. Chem., 5, 4820-4870 (2014)
The thiol-Michael addition is sometimes referred to as a “thiol-ene” reaction, which, while accurate, creates some confusion with the radical mediated thiol-ene reaction. Here “thiol-Michael” will refer to the Michael addition of a thiol to an alkene while, “thiol-ene” will refer only to the radical-mediated addition.
Z. Liu, J. Ou and H. Zou, Analytical Chemistry, 82, 89-99 (2016)
B.D. Fairbanks, D.M. Love, and C.N. Bowman, Macromol. Chem. Phys. 218, 1700073 (2017)
N.B. Cramer, C.L. Couch, K.M. Schreck, J.A. Carioscia, J.E. Boulden, J.W. Stansbury, C.N. Bowman, Dent Mater., 26 (1): 21–28 (2010)
B.D. Mather, K. Viswanathan, K.M. Miller, T.E. Long, Prog. Polym. Sci. 31, 487–531 (2006)
Michael addition reactions can also be initiated by a number of other catalysts, including metals, organometallics, Lewis acids and many nucleophiles.7
April 30, 2020