Addition Reactions of Isocyanates
Isocyanates, especially diisocyanates and polyisocyanates, are important monomers that undergo a broad range of chemical reactions. For example, they can react with compounds containing active hydrogen atoms, like amines, alcohols, mercaptanes, water, and carboxylic acids or they can react with themselves to form dimers (uretdiones) and trimers (isocyanurates) or they can be polymerized to polyisocyanates (N-substituted 1-nylons). The by far most important reaction, however, is step-growth polymerization of a diisocyanate with a polyol compound such as a polyester or polyether polyol which leads to the formation of urethane bonds. In the absence of a catalyst, the positively charged carbon of the isocyanate group (-N=C=O) is attacked by the nucleophilic oxygen of an alcohol group while its active hydrogen is added to the negatively charged nitrogen (nucleophilic addition to C=N bond). It is believed that the reaction proceeds via a four- or six-membered transition state including one or two alcohol groups.1-4Assuming a hydroxyl group acts as a catalyst, the reaction could proceed as follows:
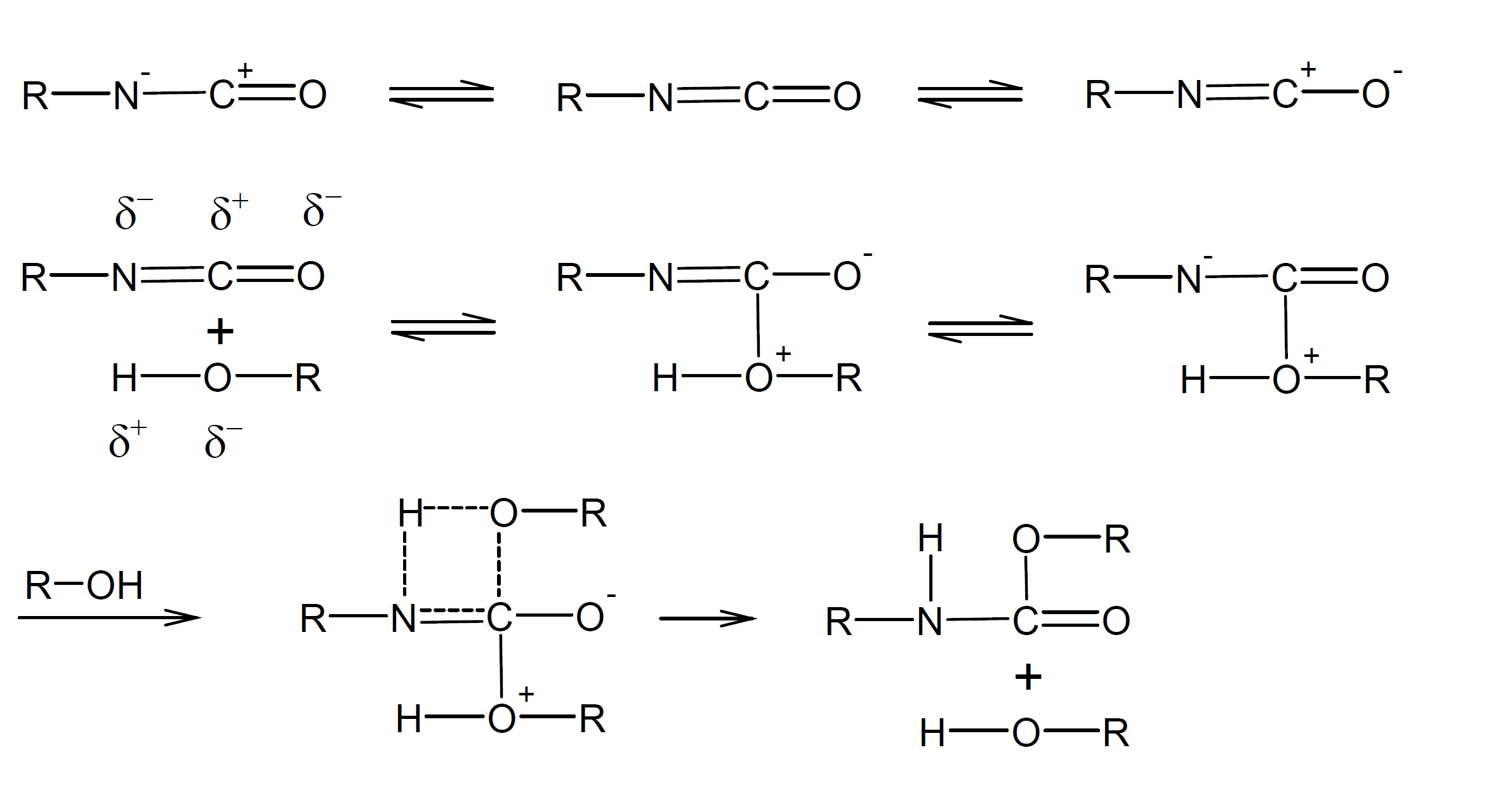
Electron withdrawing substituents increase the positive charge on the carbon of the NCO group which facilitates nucleophilic addition of alcohols whereas electron donating groups decrease the reactivity. In the case of aromatic substituents, the negative charge gets delocalized into the π electron ring system which further increases the positive charge on the carbon atom:
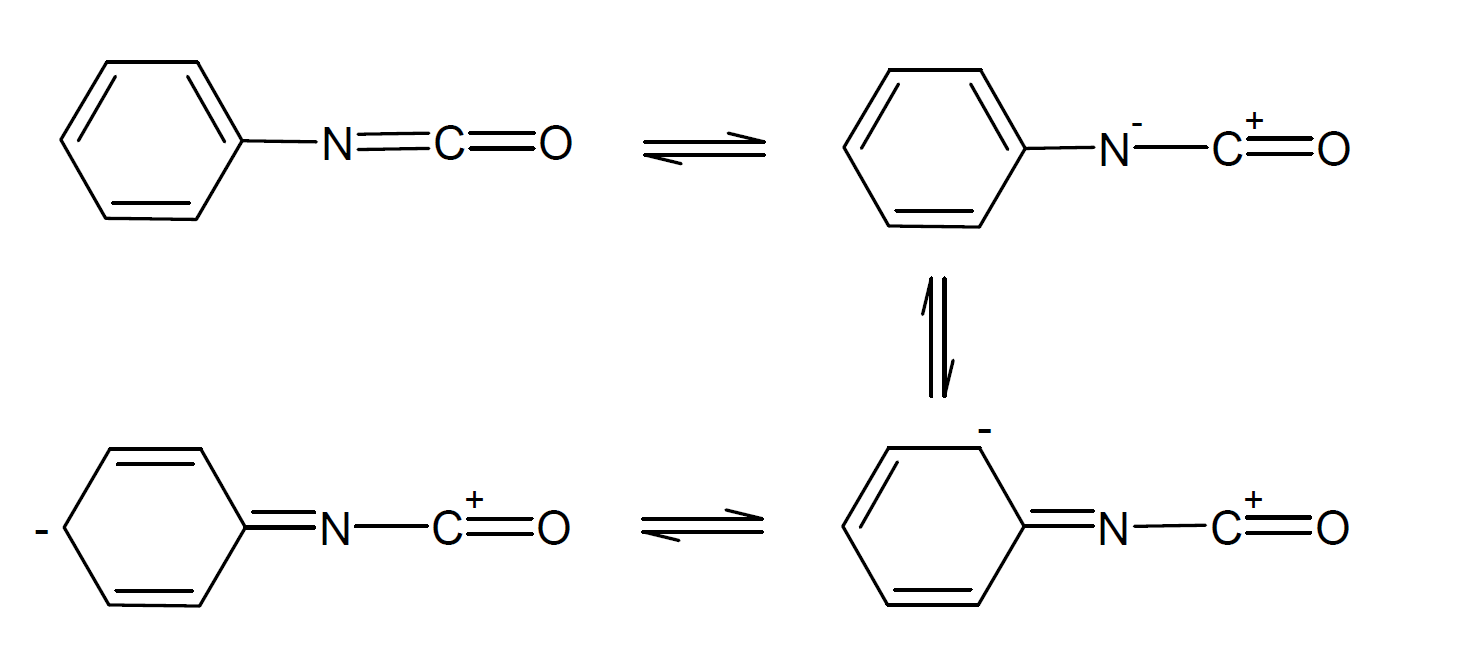
For this reason, aromatic isocyanates are more reactive than aliphatic or cycloaliphatic isocyanates. Other substituents on the aromatic ring also affect the reactivity, that is electron withdrawing side groups in ortho or para position increase the reactivity and electron donating substituents lower the reactivity of the isocyanate group whereas a second isocyanate group in ortho or para position relative to the first group increases the reactivity of the first isocyanate. This is particularly true for diisocyanates in para position whereas diisocyanates in ortho position have somewhat lower reactivity due to steric hindrance between the two NCO-groups. The same is true for other bulky side groups in ortho position.
The rate of addition also depends on the type of nucleophile; the order of reactivity in uncatalyzed isocyanate (polymerization) reactions typically decreases in the order: primary aliphatic amines > secondary aliphatic amines >> aromatic amines > primary alcohols > water > secondary alcohol >> carboxylic acid > ureas >> urethanes.3
Isocyanate reactions are very susceptible to catalysis that is catalysts greatly increase the rate of nucleophilic addition of compounds with active hydrogen to the C=N bond. Widely used catalysts include tertiary amines, such as triethylene diamine (TEDA), dimethylpiperazine (DMP), dimethylethanolamine (DMEA), and diazabicyclooctane (DABCO), as well as tin compounds like dibutyltin dilaurate (DBTL). The latter are much stronger catalysts than tertiary amines, i.e. only very small amounts are required to accelerate the additon reaction.5 These compounds polarize either the isocyanate or hydroxyl compound and thus make the C=N bond more susceptible to nucleophilic addition of the hydroxyl group.3 In the case of tertiary amine catalysts, it is believed that the tertiary amine and isocyanate form an activated complex which facilitates the nucleophilic addition of the alcohol to the N=C double bond:6,7,11
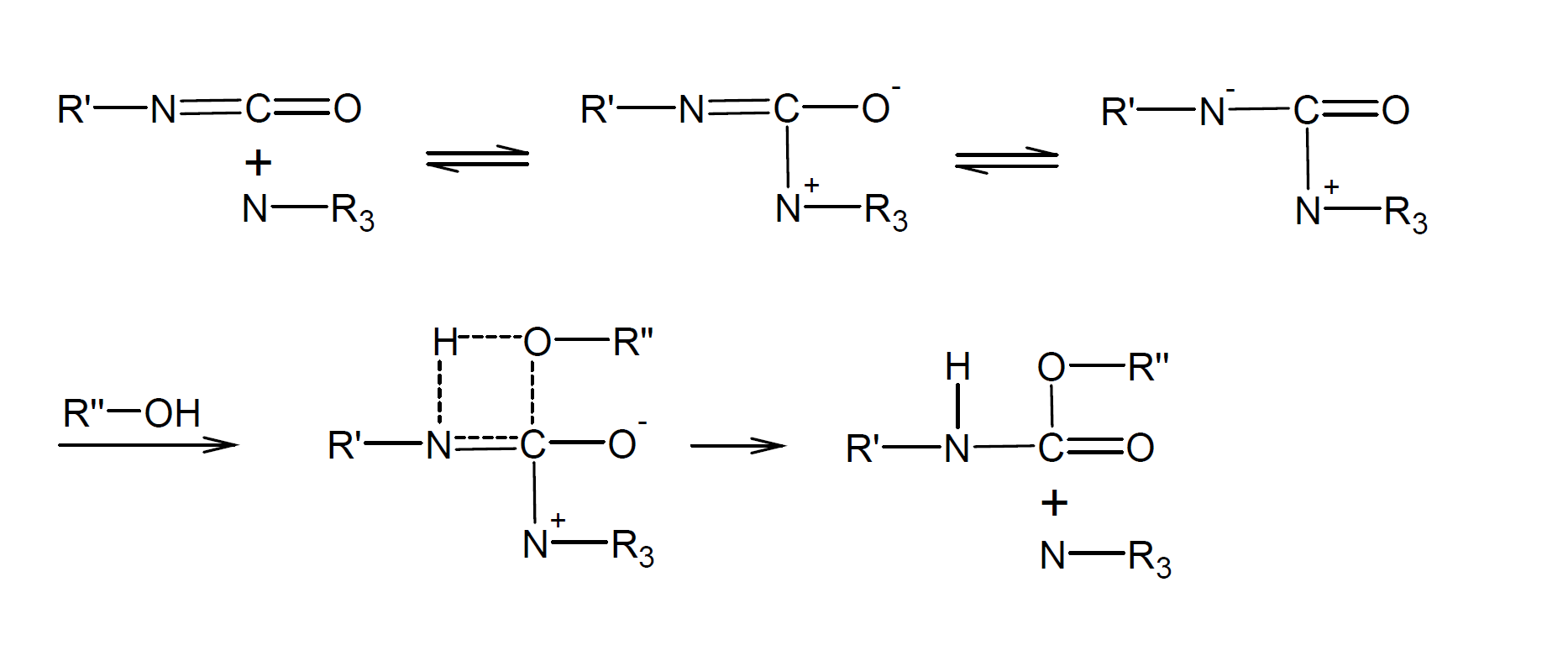
The catalytic activity of amines is more or less proportional to its base strength except when steric hindrance interferes with the formation of the intermediate state and the addition of the alcohol.
Isocyantes can undergo many other reactions. In some of these reactions carbondioxide (CO2) is released. For example, isocyanate groups react with water to unstable carbamic acid intermediates (-NH-C(=O)-OH-) which immediateley decompose to amine and carbon dioxide. The latter can act as a blowing agent whereas the amine reacts with isocyanate to urea (-NH-C(=O)-NH-). Some of the most common addition/condensation reactions are depicted in the Table below.
| Reaction | Product |
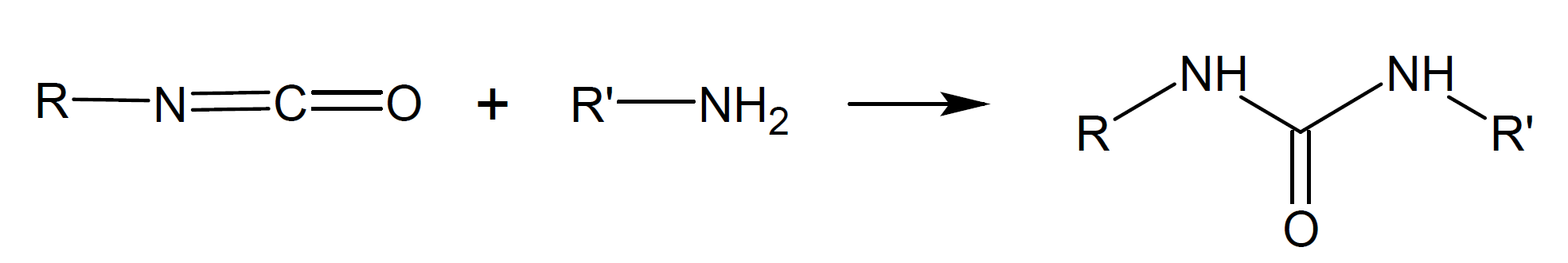 |
Urea |
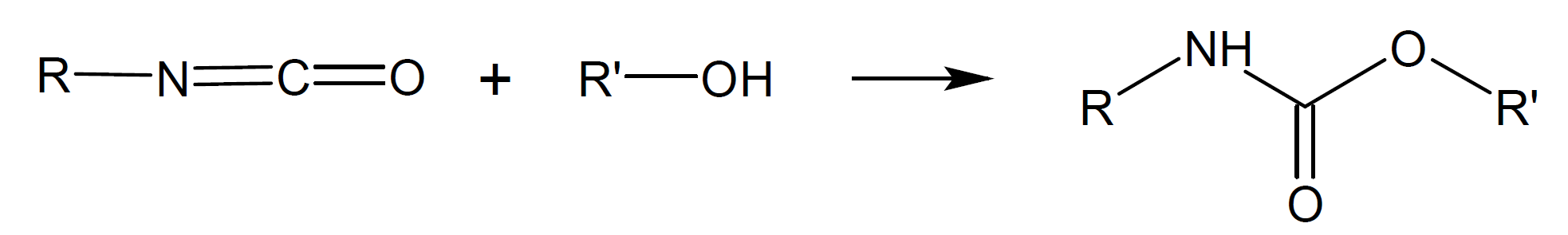 |
Urethane |
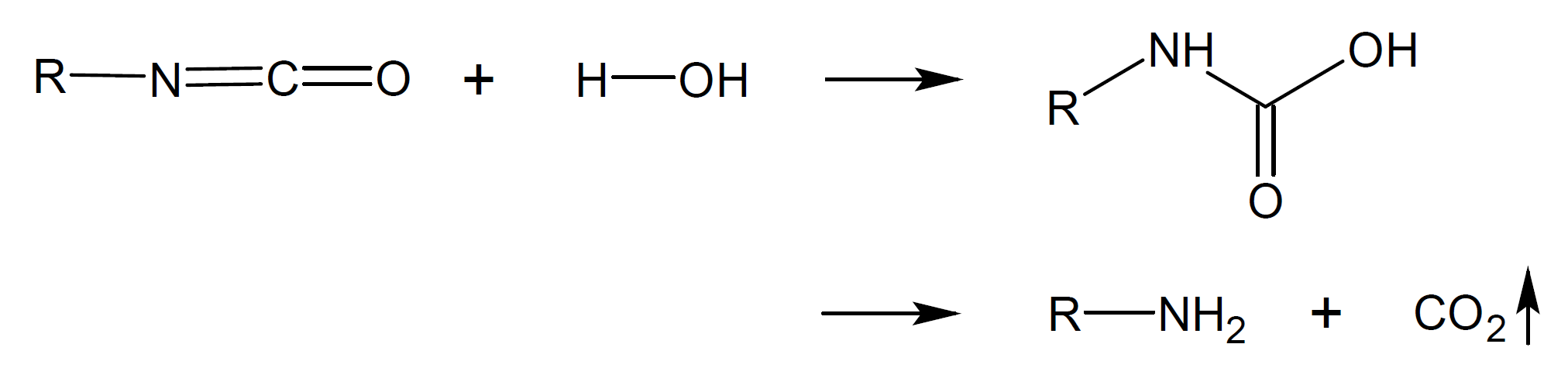 |
Amine |
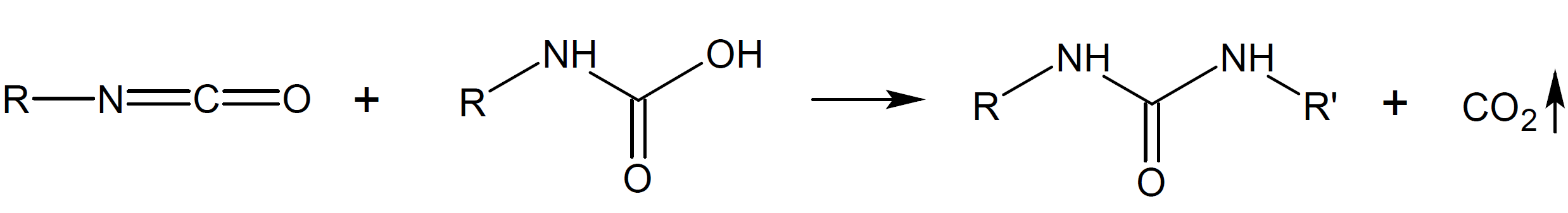 |
Urea |
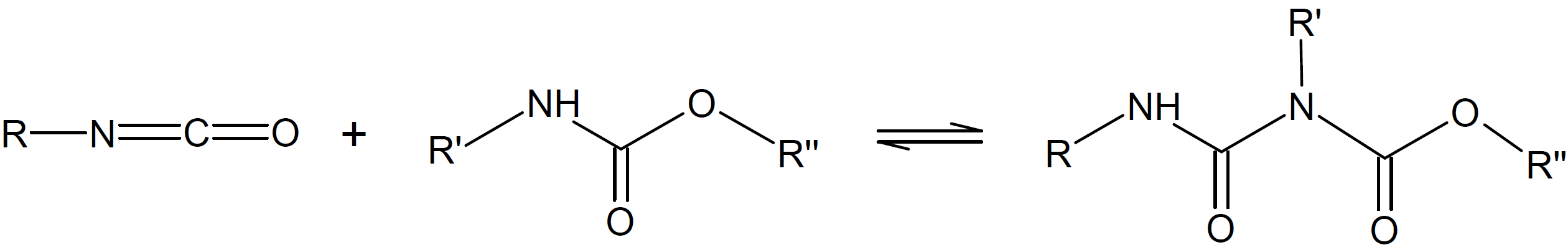 |
Allophanate |
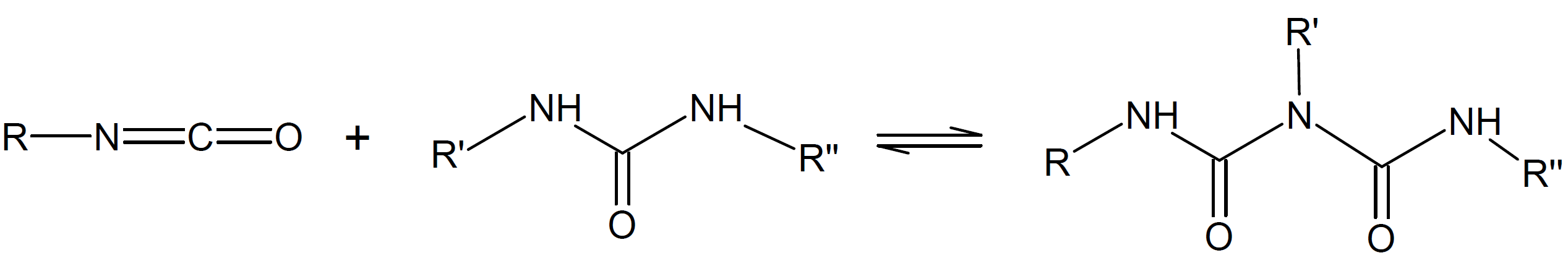 |
Biuret |
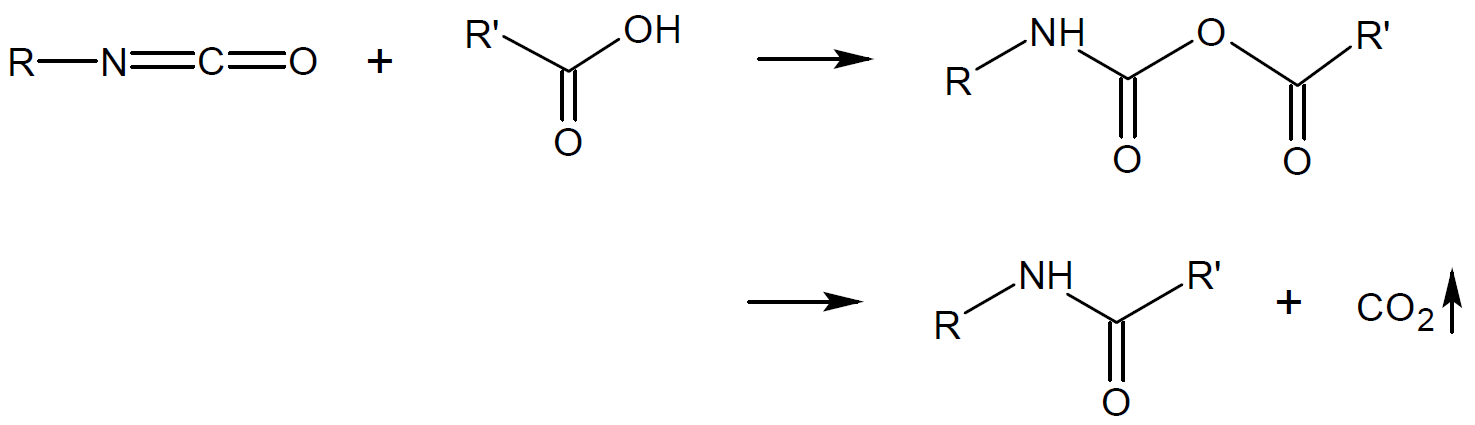 |
Amide |
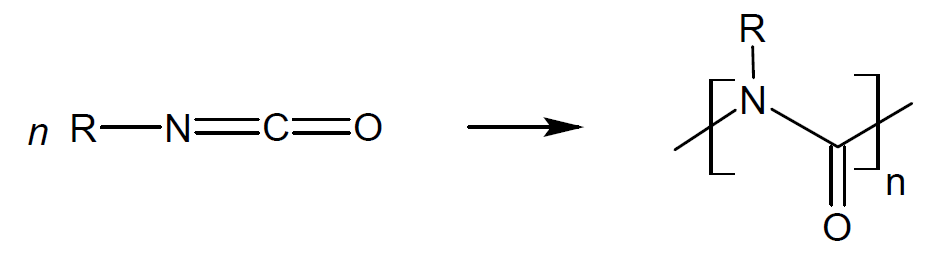 |
1-Nylon |
The most important commercial isocyanates are diphenylmethane diisocyanate (MDI), toluenediisocyanate (TDI), hexamethylene diisocyanate (HDI) and their polymeric forms. MDI and TDI are cheaper and more reactive than aliphatic isocyanates (HDI) whereas aliphatic isocyantes yield polymers that are more UV-stable (do not discolor) and are less susceptible to oxidation and degradation.
The most important class of isocyante polymers are polyurethanes. They are produced by step-growth polymerization of (polymeric) isocyanate with a polyester or polyether polyol:
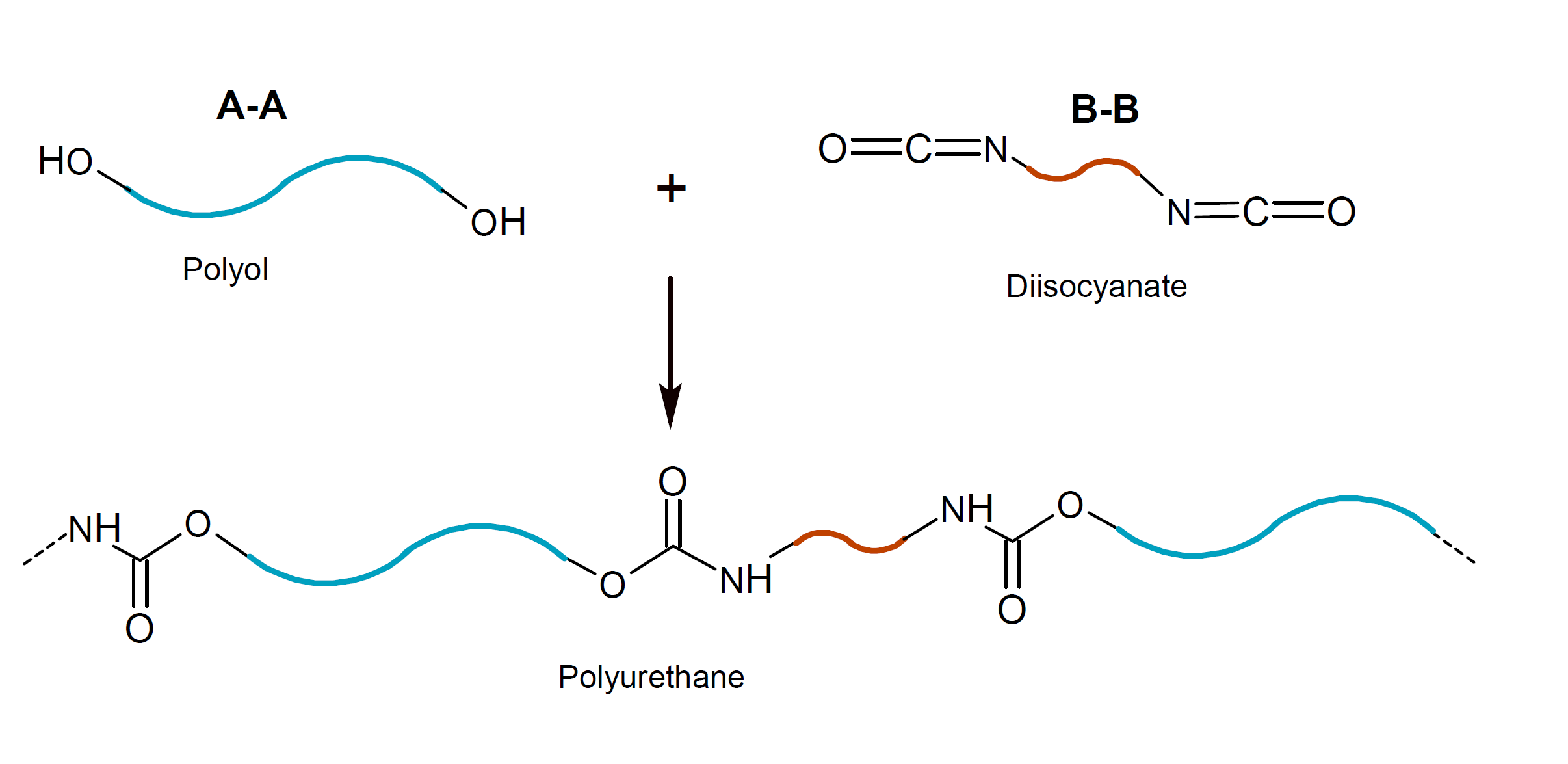
They typically consist of long polyether or polyester blocks linked by short urethane blocks. The urethane groups (-NH-C(=O)-O-) form strong intermolecular hydrogen bonds between the hydrogen of the secondary amine group and the carbonyl oxygen which act as physical crosslinks whereas the aliphatic diol portion forms only weak bonds. The properties of a polyurethane depend to a larger degree on the type, molecular weight and hydroxyl number of the polyol.10 Two very common polyols are hydroxyl-terminated polypropylene oxide (PPO) and polytetramethylene oxide (PTMO). Both polyols have low melting points and very low glass transition temperatures. Thus polyurethanes based on these polyols are rather soft and flexible.
Notes & References
Due to resonance, the carbon atom of the isocyanate group is positively charged making it very sensitive to nucleophilic attack.
K.C. Frisch and L.P. Rumao, J. Macromol. Sci., Part C, Poly. Rev., Vol. 5, 103-149 (1970)
M.F. Sonnenschein, Polyurethanes - Science, Technology, Markets, and Trends, The Dow Chemical Company, Midland, MI, USA, 2015
G. Raspoet, M.T. Nguyen, M. McGarraghy, & A. Hegarty, J. Org. Chem., 63, 6878-6885 (1998)
Other metal compounds from bismuth, zinc, zirconium, mercury, and lead (mostly fatty acid salts) also catalyze the isocyanate-alcohol reaction but are less frequently used. In some cases, a mixture of two catalysts is even more potent and in other cases other catalysts are more selective or allow for a lower reaction temperature. For example, zirconium based complexes produce significantly less carbon dioxide than DBTL as a result of a different mechanism of catalysis.8,9
J.W. Baker, M.M. Davies, and J. Gaunt, J. Chem. Soc., 24 (1949)
J.W. Baker and J.B. Haldsworth, J. Chem. Soc., 713 (1947)
M.S. Rolph, A.L.J. Markowska, C.N. Warriner & R.K. O. Reilly, Polym. Chem., 7, 7351 (2016)
J.O. Akindoyo, M.D.H. Beg, S. Ghazali, M.R. Islam, N. Jeyaratnama & A.R. Yuvarajc, RSC Adv., 6, 114453 (2016)
The average functionality of polyols varies typically between 2 and 8. For most applications (elastomers), however, a low functionality close to 2 is prefered.
J. Burkus, J. Org. Chem., 26, 3, 779-782 (1961)